Tracer-QC’s routine use in a clinical F18-FDG production facility
9 January 2024
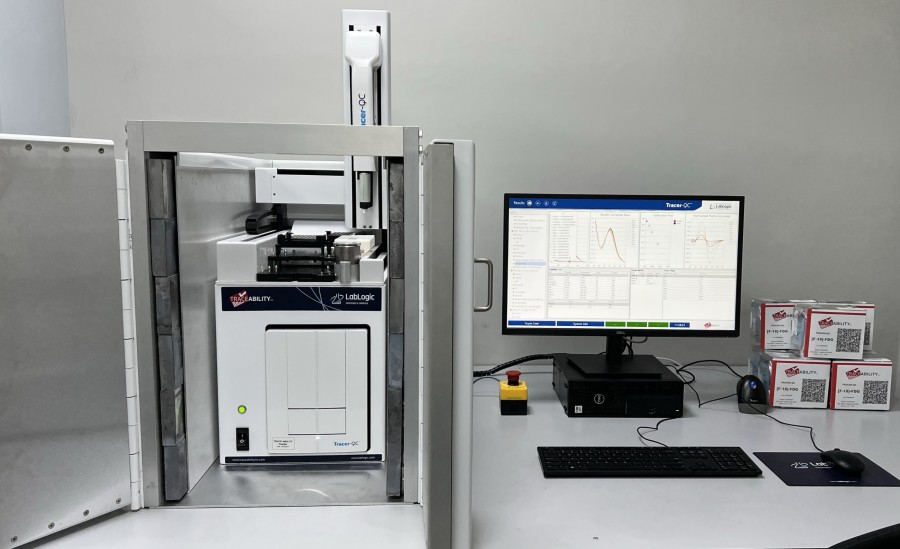
The International Medical Center in Egypt has praised the system’s automation of traditional QC release testing for PET radiopharmaceuticals
The IMC is a large tertiary healthcare hospital in Cairo which has been using Tracer-QC to successfully release 18F-FDG for administration to patients. Tracer-QC is an automated solution for the Quality Control of radiopharmaceuticals. Developed collaboratively by Trace-Ability and LabLogic, it offers 100% objective, quantitative, hands-free analysis of radioactive samples from sample to report.
The IMC chose the automated system over traditional QC equipment to release radiotracers to standards as defined by US Pharmacopeia. Its nuclear medicine department has its own PET production facility, including a cyclotron and hot lab, and the hospital has accreditation from the US-based Joint Commission International, recognising the quality and standards of its healthcare at a global level.
“It really is a significant leap forward.”
Sherif Maher of the IMC said, “Introducing Tracer-QC to the department has allowed our staff to simultaneously manage our cyclotron, tracer synthesis, and QC, which has brought about much needed savings in time and effort. As Tracer-QC is independently performing the QC, we can attend to other tasks, and simplify our operations.
“LabLogic has supported us from the very beginning with site visits for installation and staff training, which itself took less than a full day. The automated release testing is accurate and repeatable, which has given us great confidence in Tracer-QC. It has helped us modernise our practices and now we have it running, it is difficult to imagine another way of working. It really is a significant leap forward.”

Demonstrable validity of a novel solution
Speaking on this important milestone in the introduction of automation to radiopharmaceutical production, LabLogic Sales Director Elvir Zahirovic said, “It’s amazing to see Tracer-QC being used on a regular basis and delivering significant benefits in a live facility. We have been very confident in Tracer-QC for a long time but as the solution challenges the industry norms, it was always going to take time to gain acceptance and confidence. We are grateful for the support shown by the community so far and this step further demonstrates its usefulness and the validity of the system. We are extremely excited about the potential for Tracer-QC.”
Successfully introducing automation to PET production
Trace-Ability Director and Founder Arkadij Elizarov said, “This is a significant marker for Tracer-QC, not only for us at Trace-Ability and LabLogic, but for the industry as a whole. We are very proud to be the first company to deliver on getting a fully automated QC solution up and running in a clinical PET production facility.
“We also hope this news brings confidence that automation can be successfully introduced, and that Tracer-QC delivers on its promises. We have worked tirelessly over the years to optimise the system and get it to a position where it is reliable and consistent. We have proved that time and time again with our own testing, but now to see it happening in a real-world environment is very satisfying.”
Find out more
You can learn more about Tracer-QC and its automation of the PET-QC process by clicking the button below to speak to a product specialist.