Eliminating paper records in PET/QC with PETra
17/01/2024

LabLogic’s Radiopharmacy Project Manager Luke Lawrence with PET Pharm Biotech General Manager Terry Lin (far right) and his team.
PET Pharm Biotech in Taiwan is using LabLogic’s LIMS to improve efficiency by digitising their batch records
PET Pharm Biotech is a PET radiopharmaceutical CDMO and the first of only two PIC/S GMP-certified companies in Taiwan, supplying hospitals and cancer centres in the capital Taipei and across the north of the island. The company has recently implemented LabLogic’s PETra software to eliminate paper records and improve efficiency in PET production.
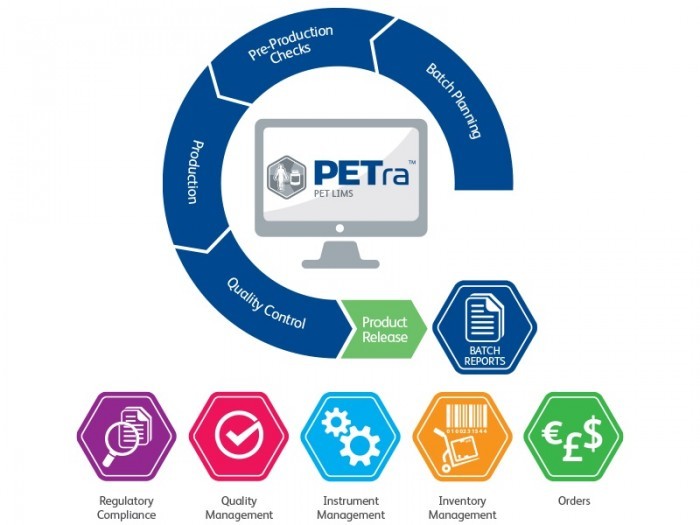
As a Laboratory Information Management System, PETra allows direct data capture by interfacing with a range of equipment and other software solutions such as Laura Radiopharma. PETra is already commonly used in radiopharmaceutical and theranostic production facilities across North America and Europe, and this most recent installation at PET Pharm Biotech is the first site in Taiwan to adopt the system.
Designed specifically for PET
General Manager Terry Lin said, “We specifically introduced PETra to improve our quality systems. Until now, we have been recording our batch records by hand to paper, which is not good for data integrity or efficiency.
“When we decided to digitise and were considering possible solutions, we evaluated other data management systems, but LabLogic’s was the most suitable, being designed deliberately for PET. Before introducing PETra, our QC lab had been using Laura for instrument control for years, so we were already familiar with LabLogic.”
“We will abandon all paper records within the next three months.”
By capturing and managing data digitally, PETra delivers significant improvements in productivity, which is what attracted PET Pharm Biotech to the system. Terry said, “PETra will reduce our manpower hours and improve our efficiency. We will abandon all paper records within the next three months. We believe the improvements that PETra will bring to PET Pharm Biotech will greatly benefit Taiwanese nuclear medicine.
“We are very confident in PETra’s capabilities, and we are hoping to speak at the next Taiwan Nuclear Medicine Society’s Annual Meeting to share our experiences with it.”
Understanding regulatory compliance
LabLogic has dedicated project managers with industry-experience who understand the rigours of regulatory compliance to successfully implement new installations. Terry praised Radiopharmacy Project Manager Luke Lawrence, saying “The team at LabLogic have been very good and Luke has been very professional. His knowledge and understanding has helped us greatly. We are planning to build a new facility, much bigger than our existing one, and will be using LabLogic’s solutions again.”
Find out more
You can learn more about PETra by clicking the button below to speak to a product specialist directly to request a guided demonstration.