LabLogic’s Laura for PET has been rebranded as Laura Radiopharma
30/11/2023
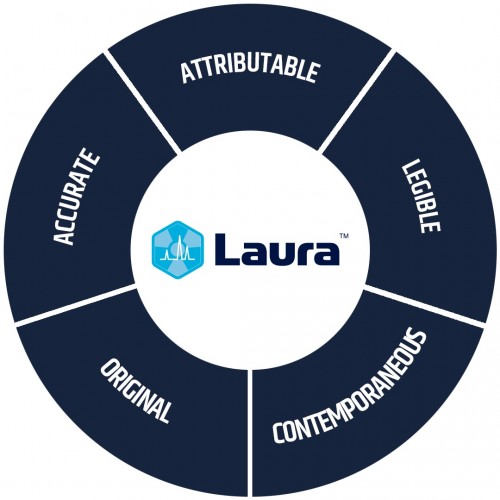
Our radiochromatography software for PET and SPECT adheres to GMP-compliant ALCOA principles
Globally, Laura for PET is used as the preferred software solution to control and analyse data from radiochromatography instruments in PET and SPECT radiopharmacies and this is now better reflected in the new name, Laura Radiopharma.
One of Laura Radiopharma’s key strengths has always been its strict adherence to ALCOA principles, which states that complete, consistent, and accurate data should be:
- Attributable
- Legible
- Contemporaneously recorded
- Original or a true copy
- Accurate
Data integrity with Laura Radiopharma
Data Integrity is critical for GMP-compliance audits. ALCOA constitutes a set of principles crucial for upholding data integrity within the radiopharmacy sector, initially introduced by the FDA and still actively employed. Laura Radiopharma software is compliant with Good Manufacturing Practices and ALCOA guidelines by ensuring data integrity through the following principles. Data integrity is carried through from Laura to the batch record when interfacing to our LIMS solutions.
Attributable
Ensuring the traceability of collected, generated, or updated data requires recording the identity of the responsible person, system, sensor, equipment, or device, along with specifying the data source and recording the date and time. While modern systems possess these capabilities, challenges mainly arise from procedural and policy aspects. Lablogic's Laura Radiopharma attributes each action to a specific user, facilitating a transparent record of task performance or data entry.
Legible
Legibility goes beyond immediate readability, especially for digitally generated data that must remain comprehensible even decades later. Laura Radiopharma generates legible and easily understandable digital records, ensuring clarity and comprehension without the need for clarification from the data originator, who may not be available anymore.
Contemporaneous
Recording data in real-time is essential to prevent delays or inaccuracies associated with retrospective data entry. Laura Radiopharma captures data as events occur in its database, ensuring contemporaneous recording and accurate timestamping without introducing operational delays.
Original
Records should be original copies and digitally recorded data requires technical and procedural measures to prevent alterations. Laura Radiopharma retains both original data and metadata, preserving the integrity of information without changes, even when copies exist.
Accurate
Records must accurately reflect what occurred, be error-free, and any changes should be thoroughly documented without losing the original information. Laura Radiopharmacy software minimises errors through validation checks and data verification processes, enhancing the accuracy of recorded information.
By steadfastly adhering to these ALCOA principles, Laura Radiopharma establishes a robust platform for maintaining data integrity and meeting regulatory guidelines across pharmaceuticals, nuclear medicine, and manufacturing industries.
Find out more
You can learn more about Laura Radiopharma by clicking the button below to speak to a product specialist directly to request a guided demonstration.