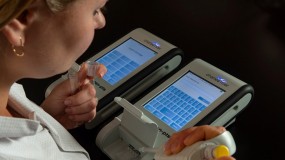
A rapid, point-of-use handheld spectrophotometer for PET-QC solutions
The Endosafe® nexgen-PTS™ by Charles River is a rapid, point-of-use handheld spectrophotometer that uses USP/BET-compliant disposable cartridges for accurate, convenient, and real-time endotoxin testing, glucan concentration determination, and Gram identification. With its small benchtop footprint, the Endosafe® nexgen-PTS™ is ideal for small to mid-size pharmaceutical companies and compounding pharmacies that want a portable, real-time testing option on the manufacturing floor.
Portable Endotoxin Testing System
The enhanced features of the Endosafe® nexgen-PTS™ address your needs for decreased assay run time, simplified data entry, reduced user variability, and enhanced administration control in your endotoxin testing. The addition of a User Management functionality allows the system to be 21 CFR Part 11 compliant-ready.
Whether you choose to perform endotoxin testing at the point of sample collection or in the central QC lab, the portability and exceptionally fast results of the Endosafe nexgen-PTS™ can enhance your LAL testing programs and accelerate your manufacturing quality control processes.
FDA-Licensed LAL Cartridge Technology
The LAL assay is the gold standard for endotoxin testing, and while conventional kinetic testing is a tried and trusted method, advances in science have allowed for improvement in how it’s utilized.
The Endosafe® cartridge technology delivers higher sensitivity and faster quantitative LAL testing results. Designed to optimize and refine the use of LAL, the cartridge technology eliminates a significant amount of the raw material and accessories required for traditional endotoxin testing methods while reducing time-consuming preparation and technician variability.
Each cartridge contains precise amounts of FDA-licensed LAL reagent, chromogenic substrate, and controls needed to obtain rapid, accurate results. The cartridges automatically perform a duplicate sample and positive product control, thereby satisfying the harmonised BET chapters USP<85> or EP<2.6.14> for LAL testing.
Q. Is the Endosafe® nexgen-PTS™ 21 CFR Part 11 compliant?
A. Yes, the Endosafe® nexgen-PTS™ is 21 CFR Part 11 compliant-ready. It is up to the end user to ensure compliance.
Q. Is there another instrument that can Endosafe® cartridges besides Endosafe® nexgen-PTS™?
A. Yes, there are two other Charles River Endosafe® endotoxin testing instruments that can be used with the Endosafe® LAL cartridge technology:
- Endosafe® nexgen-MCS™
- Endosafe® Nexus™
Only Charles River Endosafe® instruments use cartridge technology. No other brand of endotoxin testing instruments is equipped to use cartridge technology.
Q. How is sensitivity determined for the LAL cartridges?
A. Charles River supplies LAL cartridges with different sensitivities as the level of endotoxin that is allowed in specific products vary. Your choice of cartridge sensitivity will depend on the type of sample being tested, the endotoxin limit, and level of interference (how much dilution is required).
Documents
-
Solutions-for-Radiopharmacy-EU-Version-1.2
Download (19.11 MB)