Debra – A LIMS for ADME and Beyond
02/06/2020
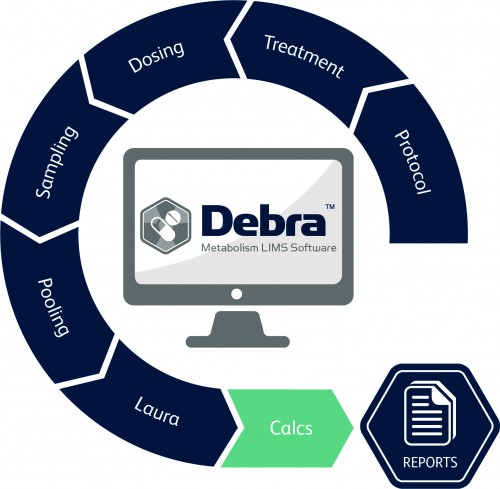
What does Debra do?
LabLogic Systems’ Debra software is a fully-featured Laboratory Information Management System (LIMS) written specifically to manage the life cycle of a range of drug and environmental metabolism studies.
It addresses the issues of time-consuming manual data entry/transcription and checking, manual calculations, assurance of data integrity and working in compliance with GLP.
Direct input from pharmaceutical/agrochemical companies and CROs
Originally written in the 1980s, Debra has been continuously developed for over 35 years with direct input from pharmaceutical/agrochemical companies and CROs around the world to meet the needs of laboratories performing metabolism studies. It has been used by the majority of the industry leaders for many years, for both GLP and non-GLP studies.
Protein binding assays, soil metabolism studies and more
Historically focused on ADME studies, Debra has expanded to incorporate many other study types, including a range of protein binding assays, soil metabolism studies and plant ‘nature of residue’ studies.
Debra is an integral part of the laboratory environment
Debra manages the entire study workflow, from protocol definition, dose formulation and administration, through to sampling, calculations and reporting. Not just an ‘add-on’ to existing processes, Debra becomes an integral part of the laboratory environment.
Built around the core principles of Good Laboratory Practice
Debra captures raw data directly from instrumentation, automatically performs all required calculations and generates final reports at the click of a button.
All data is held in a secure database so cannot be accessed outside of the software; built around the core principles of Good Laboratory Practice, Debra comes with a full security system, audit trail and electronic signatures.
What are Debra’s key benefits?
Regulatory Compliance
Studies conducted for the assessment of safety or efficacy of chemicals to man, animals and the environment are required to be performed to GLP standards. FDA principles require that complete, consistent, and accurate data should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate. This ensures the integrity of the data so that it can be relied upon in confidence.
To comply with these principles, strong processes need to be in place. Data management is a key factor not only during a study, but also after a study is complete so that data is available long after it is recorded.
Debra has been written from the ground up with regulatory compliance and data integrity at the root of its functionality. The principles of GLP have been applied throughout the application so that all the tools required to comply with regulations are available and can be easily applied.
Access to Debra is controlled via a highly-configurable security system that uses a mixture of security levels and user ID/password. This means that the areas of Debra that are accessible to users, and the activities that users can perform, can be configured on a study-by-study basis. In addition, a full audit trail means that any changes to the system or data are tracked and reported, so that there is full transparency on all actions by users within the system.
All data and settings in Debra are stored in a database that cannot be accessed externally; this means that once data has been captured, it cannot be changed or re-entered without a further record of that change. It is not possible to manually enter data, change/edit data, or alter any settings without full details of the activity being logged, including who made the change, when it was made and what the change was (the data before and after the change). This prevents any accidental alterations to data or settings, and in the worst case scenario, data manipulation/falsification.
Debra also offers the facility to require electronic signatures for actions performed within the software, in accordance with FDA 21 CFR Part 11. If enabled, users must sign whenever they perform actions that require a signature; there are also options to require an approval signature by a senior member of staff.
Direct data capture/import
One of Debra’s most important features is that it captures all weights associated with a study directly from a balance into the database. This means that raw data is not manually entered or subsequently transcribed into a spreadsheet, vastly reducing the chance of human error and also providing significant time-saving benefits.
Debra does allow data to be manually entered if this is required, but an audit entry must be entered. All data entry is fully tracked with meta-data, including details of who entered the data, a date/time stamp and workstation details.
LSC radioactivity data is imported into Debra from the counter output file and Debra can be configured to accept electronic dpm data from any liquid scintillation counter. Background and combustion efficiencies are automatically accounted for.
Debra can also import data from a range of whole body autoradiography (WBA) systems.
Automated calculations
Metabolism studies involve a variety of calculations, from dose formulation and dosing calculations, through to sampling results. Manual calculations can be prone to error and are time consuming, so data verification is of high importance.
Debra performs all calculations internally – no manual calculations or checking of data are required. Simply put, there is no faster way to go from raw data to final results than Debra. Standard calculations, such as recovery and concentration, are performed and can take account of various factors such as LOD/LOQ, high variance samples and multi-dose schedules. There are many configurable calculation options to account for different practices across laboratories, so that Debra remains flexible to the industry’s requirements.
Calculations can be run at any time – raw data collection to final results takes a matter of seconds. Need to make a change to the compound specific activity? No problem – make the change and run calculations – all of the results are updated.
Label production
A simple but time-consuming task associated with most studies is labelling of sample containers; for GLP studies, these must be uniquely identified. Debra simplifies this task by providing an automated process to create all labels for a study in a matter of minutes.
Debra comes complete with a fully-featured labelling application which allows the design and use of unlimited label templates for use within Debra. It works in conjunction with Debra so that labels automatically contain the correct information for each sample and can incorporate barcodes, images and more complex items such as date/time formatting and mathematical functions.
To create a set of labels for a study or for specific samples, it is a simple matter of selecting the samples required and printing the resulting labels.
Barcodes
All samples within Debra are uniquely identified with a barcode. This can be used to simplify collection of data by scanning pots, vials, dosing syringes etc to link the captured data with the sample. Barcodes are produced via Debra’s label generator and increase the speed and accuracy of data collection.
Debra and the Study Workflow
Metabolism studies follow a typical workflow and are managed by Debra from protocol definition through to final report production entirely from within the software. This leads to a consistent approach across staff and ensures that workflow is harmonized.
Protocol Definition
Study protocols can vary in complexity. The more complicated the protocol, the more complicated the study will be to track dosing and sampling schedules and make sure that the data is managed appropriately.
It is therefore important for a system that manages these study types to be able to cope with the entire range of protocols, from single dose studies with a small number of subjects to complex multi-dose studies with multiple dose routes and dose rates, and large numbers of subjects and samples. Regardless of the complexity of the protocol, it can easily be configured in Debra. Templates for common studies can also be created which makes creating new studies very quick and easy.
Dose Formulation
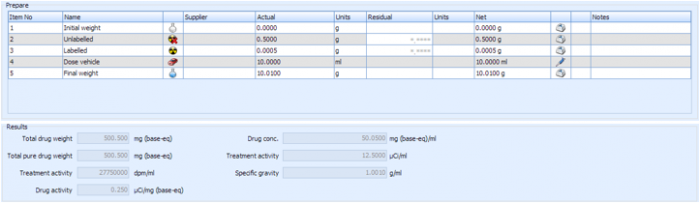
One of the most crucial parts of any radiolabeled metabolism study is the design, preparation and analysis of the dose formulation. It is critical that the formulation is prepared to provide the correct compound concentration and activity when administered to the subjects.
Debra assists with this by providing screens to guide scientists through the process, accounting for many factors such as compound purity and salt/base ratio, and calculating the amount of labelled/unlabeled compound and dose vehicle required for the formulation. Debra also assists with the preparation and analysis of the formulation by capturing compound weights/volumes, performing dilutions for analysis of the solution and capturing the resulting LSC data. Final formulation results such as specific activity and compound concentration are automatically calculated.
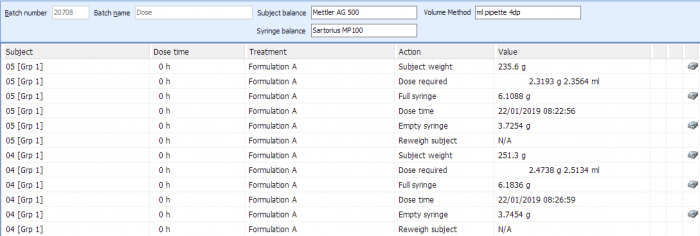
Dosing
Debra provides a variety of options to administer dose formulations to subjects. Dosing screens are interactive and prompt for the correct amount of formulation to administer to achieve the required dose rate based on the weight of subject, with results immediately calculated following each dose administration.
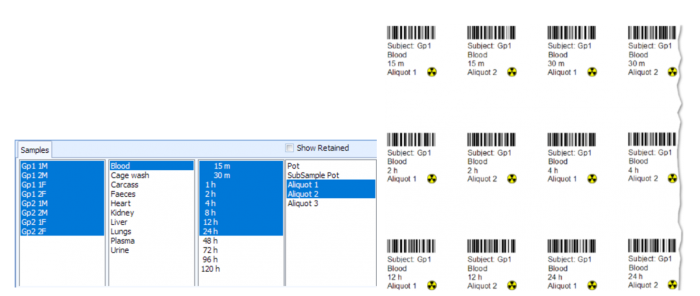
Sampling
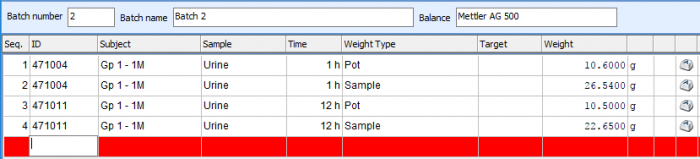
The majority of the in-life phase of a metabolism study is associated with sample collection and analysis. This can be very time consuming, with large numbers of samples requiring processing and weights recorded. Debra is specifically designed to cope with the varying sampling processes that each laboratory has; its flexibility allows samples to be processed however is required, with fully configurable options of weights for pots, samples, subsamples, homogenates, cones and aliquots.
Dealing with a high number of samples is made easy in Debra. Worksheets for weight and LSC capture can either be configured ahead of time for subsequent capture of data, or worksheets can be built up in real time via barcode scanning of samples, and all data is available at a glance to review what samples still need to be weighed. Weights are captured directly from the balance into the database at a click of a button, and LSC data is imported directly from counter output files, saving time and removing the possibility of transcription errors.
Pooling and Analysis
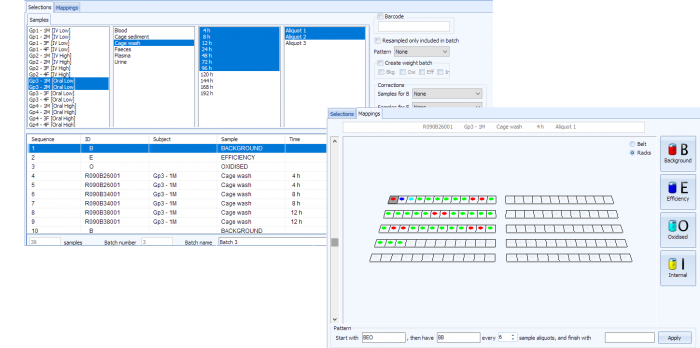
Once samples have been collected and recovery/concentration data calculated, it is common to create sample pools for analysis by chromatography. Pools can be created and prepared within Debra, based either on a percentage of sample weight or area under the curve calculations.
If LabLogic’s Laura radio chromatography system is being used for analysis, this can be taken a step further via a secure database link between Debra and Laura. Regions of interest in chromatograms can be related directly back to sample data so that parent compound and metabolite recovery/concentration can be reported from within Debra.
Reports
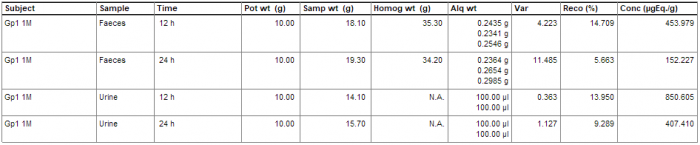
Each study requires submission of a final report including an overall summary of the result and presentation of raw data and calculated results.
Debra provides a range of reports covering all areas of a study. All raw data is available for reporting, as well as all calculated and statistical data, and final summary reports. These are immediately available following data capture so it takes minutes to go from raw data to production of a final report. If any data needs to be recaptured or calculation parameters changed, this isn’t a problem – it takes just a few minutes to make the change, run calculations and regenerate the report.
Further Links
As well as linking with LabLogic’s Laura radio chromatography software, Debra also has links with:
- Seescan WBA software: WBA studies can be configured and dosed in Debra and then the sample list exported to LabLogic’s Seescan WBA software; concentrations are then imported straight back into Debra for final reporting. This makes it easy to perform WBA studies alongside other study types.
- Stacy sample and radioisotope tracking software: allows Debra sample locations to be tracked, as well as radioactivity content. This ensures that local radioactivity rules are enforced and that site limits are not exceeded.
A Range of Studies
Although originally written for the traditional ADME study, Debra now encompasses a range of study types so that Debra’s regulatory compliance and time saving features can be of greater benefit across laboratories.
Protein Binding
Complementing ADME, protein binding studies play an important part in determining the distribution and pharmacokinetics of a drug candidate. Debra provides a range of protein binding assays to cover the most common assays, including non-specific binding and time to equilibrium options:
- Ultrafiltration
- Ultracentrifugation
- Equilibrium dialysis
- Blood cell partitioning
As in all of Debra’s study types, all required calculations (eg. %free and %bound) are automatically performed so compiling reports that contain the necessary data is quick and easy.
Environmental Metabolism
For laboratories that perform soil metabolism studies, there are additional requirements to be considered. Soils need to be characterized and moisture contents recorded and maintained, and dispensed accounting for wet/dry soil weights.
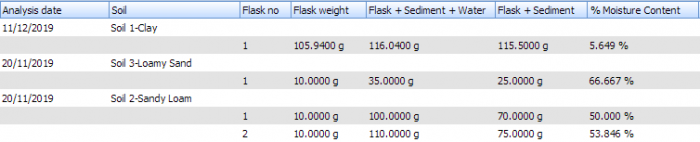
Debra provides for this, as well as the specific requirements and calculations associated with soil adsorption/desorption studies.
Nature of Residue Studies
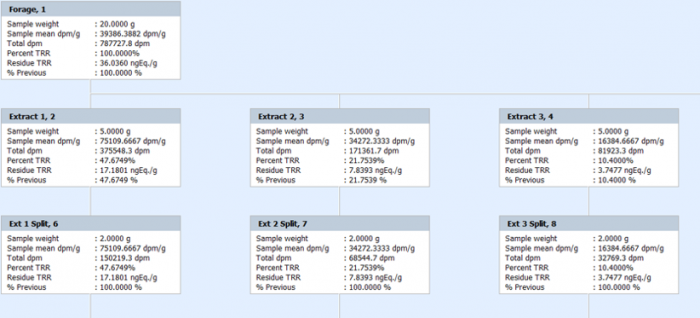
Some study types involve simple or complex extraction procedures, required to characterize residues. Debra contains functionality to create extraction trees for any sample on the fly, visually tracking progress and calculating recovery at each stage. This allows samples to be extracted, pooled, partitioned, concentrated etc, using all of Debra’s data capture and calculation functionality to continue to ensure data integrity.
Extraction trees can be created for any sample in Debra but are especially useful in plant nature of residue studies where % Total Radioactive Residue is calculated and tracked at all stages of the process.
Conclusion
Any laboratory that performs radiolabeled metabolism work, whether ADME, protein binding, soil metabolism or plant metabolism studies, recognizes the importance of ensuring the integrity of their data. With ever-increasing scrutiny by regulatory authorities, it is more important than ever to have systems in place that makes sure that data is secure and compliant with guidelines.
Debra has been used for over 35 years in GLP-compliant environments, and has been proven across many major pharmaceutical/agrochemical companies and CROs around the world to not only be a significant time saver, but also to have all the features required to comply with GLP guidelines.
Why not ask for a demo and see what Debra can do for you?