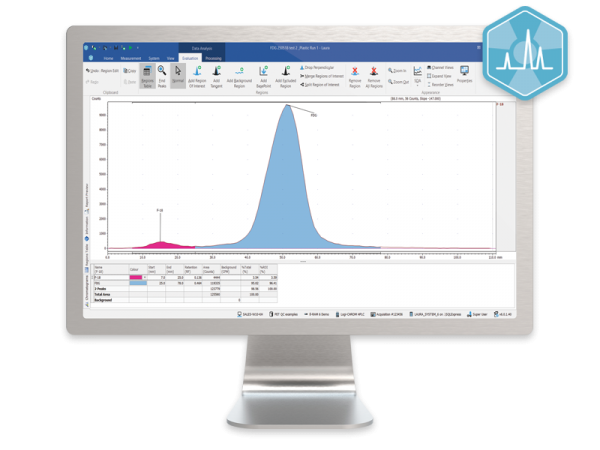
The ideal solution for PET/SPECT radiopharmacy QC.
Laura Radiopharma offers a single software solution for control, data collection and reporting from radio-HPLC detectors, radio-TLC scanners, MCA, GC and HPLC systems.
Users only need learn one software system for the complete suite of QC instruments for PET/SPECT production but Laura Radiopharma also has the analysis power to support the needs of the research and development team as well.
Laura Radiopharma also offers:
-
Regulatory compliance
-
Ability to lock methods, reports etc to ensure consistency of data
-
Half life correction
-
Relevant values reported such as % total for purity checks
-
Units of measurement correct for radioactivity, DPM, cps…
-
Simple user interface for ease of use my multiple users
Easy to use
Laura Radiopharma uses recognisable formats and wizards for multi function tasks to make it simple to use by multiple users with different levels of experience with the system.
A single software solution for the range of disciplines needed in the QC department makes the learning process easy and the day to day use effective.
Compliant
Laura Radiopharma can be installed and configured to meet the most rigorous regulatory compliance. Featuring configurable audit trail, multi level security, e-signatures and data storage into a secure database environment Laura Radiopharma is the PET/SPECT industry's answer for regulatory compliance.
Connectivity
Laura Radiopharma provides connectivity to the widest range of detectors and chromatography systems. With a wide range of detectors supported including the Flow-RAM and Scan-RAM for radioactivity detection along with chromatography systems for HPLC, TLC, GC as well as multi channel analyzers for nuclide identification Laura Radiopharma is truly the data system for all your chromatography requirements.
Single Software Solution
Laura Radiopharma is a single software solution for the PET/SPECT QC environment. Rather than having several software systems to use the QC analyst can use Laura for PET for R-HPLC, R-TLC, GC and MCA analysis. With control of both detectors and separation systems, Laura Radiopharma does it all.
Half life correction
Laura Radiopharma has the ability to correct for half life decay either as the run is in progress or post run using a defined time and date. With a comprehensive library of nuclide half lives Laura Radiopharma is designed with radioactivity at it’s core.
Regulatory compliance
Laura Radiopharma is designed to meet the regulatory compliance for GxP and FDA 21 cfr Part 11 and FDA 21 cfr Part 212 requirements. With the ability to control access, define methods and reports that can then be locked against modification the system can be configured to meet the most demanding regulatory requirements.
Complete workflow solution
Laura Radiopharma will guide the analyst through the complete QC procedure. Methods can be pre-defined and chosen from a drop down list. It is a single analysis batch for both detector and separation system and the system can be configured to auto detect and label peaks. A report is then automatically produced at the end of the run.
Why does Laura Radiopharma controlling all my QC instruments benefit me?
By having just one software system Laura Radiopharma supporting all the QC suite of instruments you have consistency of methods, batches and reporting plus having one easy to use system makes the process simpler.
Which instruments does Laura Radiopharma support?
Laura Radiopharma supports a wide range of instruments including radio detectors for HPLC, TLC and GC plus separation systems for HPLC, TLC, GC along with multi channel analyzers. Please contact us for more information about supported systems.
Can Laura Radiopharma correct for half life decay?
Yes, Laura Radiopharma can correct for half life decay either in real time as the run progresses or from a defined date and time.
Can methods and report be locked against modification?
Yes, methods, gradients and reports can all be locked against modification by the system manager. This ensures consistency of QC data.
Does Laura Radiopharma have an audit trail?
Yes, Laura Radiopharma can be installed to provide regulatory compliance and this includes a comprehensive audit trail function.
Can Laura Radiopharma provide a report automatically?
Yes, Laura Radiopharma can be configured to automatically integrate the chromatogram and produce a report automatically with values such as % total reported for purity checks for example.
Existing users of Laura Radiopharma
Click on the tabs below to find out more about our customers and how they are using Laura Radiopharma in their facilities.
St. Jude Children’s Research Hospital & University of Virginia, USA
Counting PET biomarkers to track microglial activation
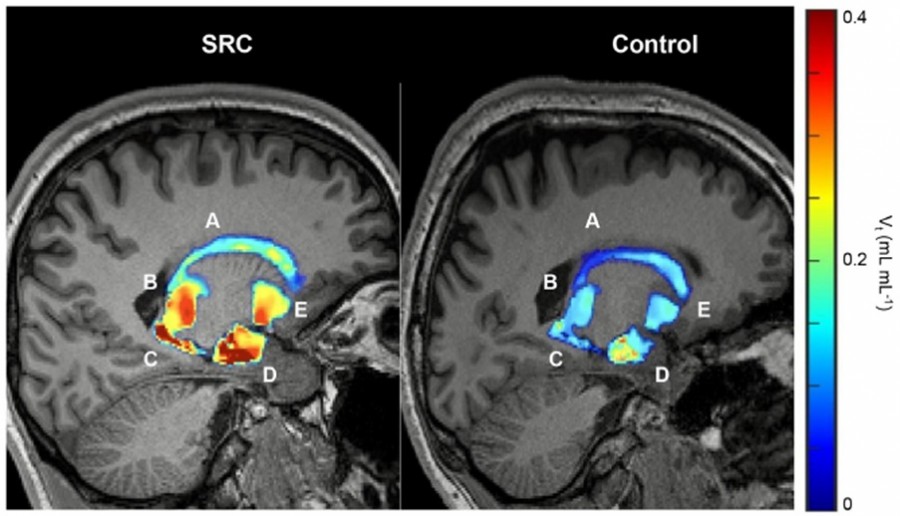
Volumetric maps quantify persistent microglial activation in athletes diagnosed with SRC who have achieved return to play compared to control groups. (A) Caudate. (B) Thalamus. (C) Putamen. (D) Hippocampus. (E) Amygdala.
The Posi-RAM PET metabolite radio-HPLC detector controlled by Laura has been used in a collaborative study by Neumann et al. between St. Jude Children’s Research Hospital, Memphis TN and the University of Virginia, Charlottesville VA. The study investigated basal levels of translocator protein (TSPO), thought to increase during microglial activation as a consequence of sport-related concussions (SRC). Because of its high sensitivity and low background, the Posi-RAM was used to quantify [18F]DPA-714 binding in the brain of college athletes following a concussion, compared to healthy subjects.
You can learn more about the study and why the Posi-RAM's coincidence counting makes it an ideal instrument for human clinical trials by clicking the button below to find out more.
Universitätsklinikum Essen, Germany
Synthesising and validating [18-F]PARPi

A Logi-CHROM HPLC system controlled by Laura Radiopharma has been installed at Essen University Hospital’s Clinic for Nuclear Medicine. Anna Pacelli carries out postdoctoral research at the radiopharmacy into the automation and validation of radiopharmaceuticals for clinical use, specifically [18F]PARPi.
Anna said, “Using Laura to control the instruments is much more user-friendly than other software I’ve used. It’s easy to set up a new methodology, start a new project or navigate through existing ones, and purge the system when I need to. Everything is displayed clearly and it’s easy to navigate. Being able to use Laura for both HPLC and TLC instrumentation is convenient and allows us to standardize our work, rather than using two different software packages.”
You can find out more by clicking the button below.
Edinburgh Imaging Facility, UK
Radiochemical purity testing for GMP and research PET tracers

LabLogic’s Scan-RAM radio-TLC scanner is being used at Edinburgh Imaging Facility’s radiochemistry unit and is controlled by Laura Radiopharma. Based at the University of Edinburgh, the unit houses a cyclotron with supporting radiochemistry suites to produce research PET radiotracers including F-18, C-11, and O-15. This allows EIF to conduct PET imaging experiments, develop fully translational tracers, and adopt existing radiotracers to GMP standards for clinical studies.
QC Manager Kenneth Wilson said, “All instrument control is via Laura software with full audit trails and electronic signatures available for GMP compliance. It offers comprehensive control of instrument parameters with saved methods and individual password-protected user logins with configurable access permissions ensuring validated methods remain safeguarded against unauthorised changes.”
You can find out more by clicking the button below.
Center Hospitalier de Valenciennes, France
Sensitive gamma spectrometry for Ga-68
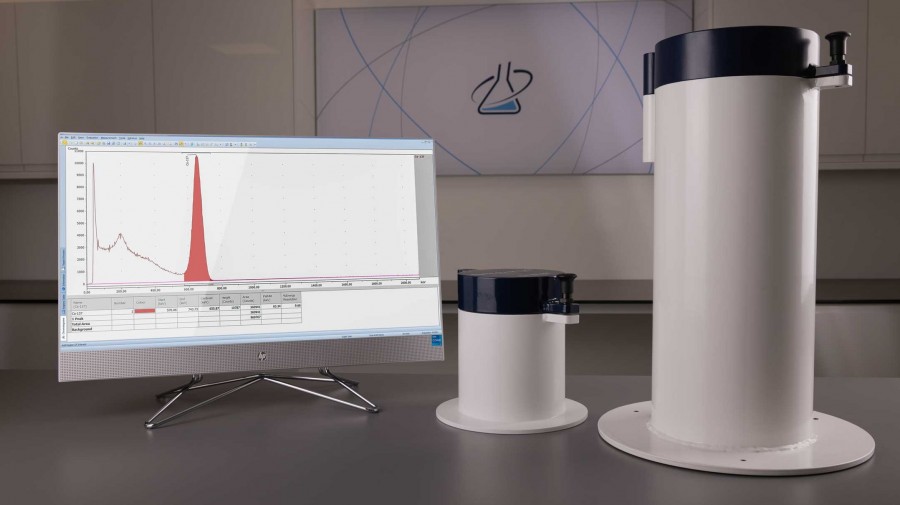
LabLogic’s Spec-RAM gamma spectrometer controlled by Laura Radiopharma has been successfully installed at the Center Hospitalier de Valenciennes in France where it is being used in the quality control of radiopharmaceuticals labelled with gamma-emitting radionuclides. When considering a replacement for their existing well counter, the compatibility of Laura with Valenciennes’ existing instrumentation proved critical.
Radiopharmacist Emmanuel Malek said, “The choice of Spec-RAM was based on its technical characteristics, in particular its size, sensitivity, and energy resolution. But the decisive factor was Laura, which we were already using for HPLC and radiochromatography. This meant that we could use just one software for all our QC equipment, making it quick and easy for our teams to get to grips with. What's more, with a remote license for Laura, I have access to all the acquisitions, whatever the equipment used, from a single software package.”
Click the button below to find out more.
Örebro University Hospital, Sweden
Managing a new PET production facility and integration with PETra
Örebro University Hospital in Sweden has chosen LabLogic’s solutions for its new radiopharmacy which is being built to supply its current and future PET/CT cameras with tracers radiolabelled with Fluorine-18 initially and eventually Carbon-11, Gallium-68, and Oxygen-15. The hospital’s radiopharmacy will use LabLogic’s PETra PET Laboratory Information Management System to manage the production and administration of PET radiopharmaceuticals along with Laura Radiopharma.
QP and Head of Quality Assurance Lisa Holm said, “We like Laura's direct data capture from the instruments. We don’t have to write things down only to copy them over to the LIMS. The integration with PETra allows us to remove the human interaction, eliminate transcription errors, and be GMP-compliant.”
You can find out more by clicking the button below.
Evergreen Theragnostics, USA
Choosing LabLogic's integrated solutions

Evergreen Theragnostics is a US-based radiopharmaceutical Contract Development and Manufacturing Organization (CDMO) that has chosen LabLogic as their partner of choice for LIMS and QC equipment controlled by Laura Radiopharma. The company offers a broad range of services for centrally distributed radiopharmaceuticals from Phase 0 through commercialisation
Head of Project Management Kevin Staton said, “We love Laura software for controlling hardware and collecting data in the QC lab, particularly due to its compliance with the FDA 21 CFR Part 11. It’s clear to us, and to auditors, how the data is stored and managed.
“Honestly, if Laura could control absolutely everything in our QC lab, we would happily use it. Having a few, centralised software platforms for data acquisition and management makes management easier for our IT department, our Quality department, and for user training. It also makes it easier for us to demonstrate compliance during external audits. For this reason, we are hoping LabLogic continues to develop even more integrations to support additional equipment in our QC lab."
You can find out more by clicking the button below.
Documents
-
Laura-Radiopharma-US-Version-1.5
Download (1.69 MB) -
Laura-Radiopharma-FR-Version-1.5
Download (1.74 MB) -
Laura Target Platform
Download (469.56 kB) -
Solutions-for-Radiopharmacy-EU-Version-1.2
Download (19.11 MB)