A key feature of PETra is the system’s ability to interface with all the key instruments and data systems from the complete production process. Each piece of equipment, to which PETra connects to or interfaces with, is automatically registered within PETra. Arrange of tools are provided in order to help manage the instrumentation.
Instrument Maintenance
To ensure that the downtime of each piece of equipment is minimized and that the facility operates at the best possible efficiency, it is essential that the equipment used is regularly maintained, and that users are notified in advance of when the required maintenance is due in order for them to plan their production schedule around that. In PETra, this process is automated and records for each piece of equipment can be fully maintained based upon a number of key parameters, namely; Supplier, Instrument Name, Instrument Type, and Frequency of the required maintenance or tests. The frequency may be defined as manually, week days, weekly, monthly, quarterly, annually or biannually and notifications set up accordingly.
Instrument Interface
This module lets the site manage the specific make / model and interface methodology for all instrumentation and interface systems A wide range of Cyclotron, Synthesis Modules, Dose Calibrator, Endosafe PTS, radio-HPLC, GC, radio-TLC, MCA, TLC Visualizer, pH meters, balances, Osmometers, Turbidmeters, Automatic dose dispensing systems, etc… All can be automatically captured directly to PETra, no manual transcription, and therefore no transcription errors.
Instrument Trending
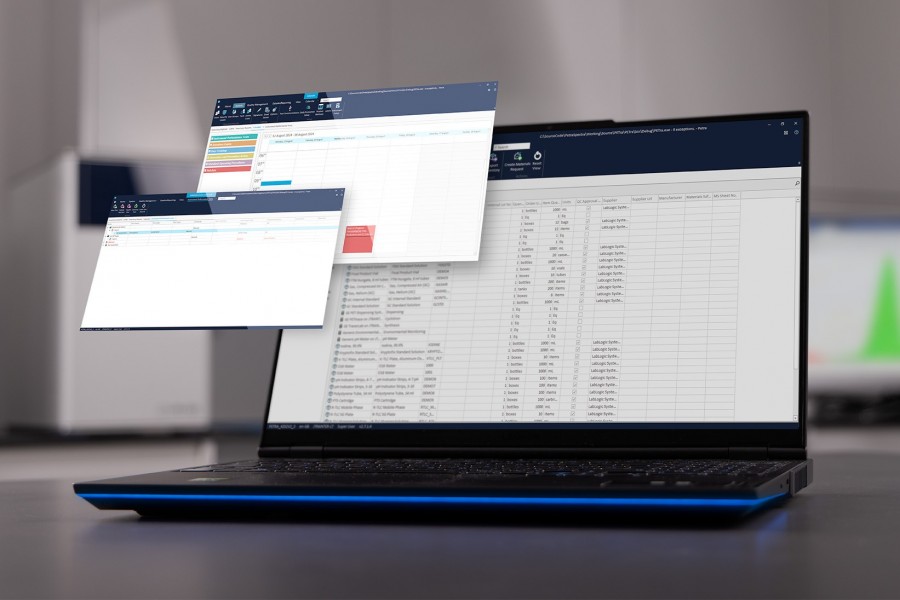
Any function can be analyzed over time to look for variations in instrument performance or any other parameter. The data is displayed graphically and in a tabulated format with quick links whereby clicking on any point will take the reviewer to the relevant section of the batch in question. This provides for rapid review of instrument performance and allows operators to identify early warning signs of requirements for equipment maintenance.