Debra for eFate News

The world’s leading LIMS for radiolabelled eFate and plant metabolism studies
04/09/2025
Find out how Debra supports a range of OECD guideline study types in this short video

Did you know Debra can create a single report to show how metabolite concentrations and recoveries change over time?
09/12/2024
Keeping track of metabolite data over the course of a metabolism study can be challenging but Debra's new HPLC Summary Report provides a solution

The Beta-RAM 6: Setting a new standard in radiolabelled metabolite detection
01/07/2024
In our latest video, we demonstrate our radio flow detector for ADME and eFate research

Managing environmental metabolism studies in an FDA/GLP regulated environment
17/03/2022
LabLogic's Debra is a LIMs designed to manage the life cycle of a range of drug and environmental metabolism studies

Manage your entire study workflow with Debra
10/06/2021
Used at major Contract Research Organisations and Pharmaceutical/Agrochemical companies around the world, Debra is the industry standard in its field, click to find out more about its key benefits.

Interested in learning more about performing eFate/soil studies in Debra?
20/08/2020
Debra LIMS for eFate Webinar: Register your interest.

Debra - Not just for ADME
13/08/2018
Debra Metabolism LIMS has been the industry standard solution to managing the entire life cycle of a range of drug and environmental metabolism studies within an FDA/GLP regulated environment. With over 30 years of development, the system has been adopted by many of the world's leading pharmaceutical, agrochemical and contract research organisations. Click here to find out more.

A LIMS ideal for Agrochemical and E-Fate studies
15/05/2018
Debra, the worlds leading metabolism Laboratory Information Management system is now also ideal for agrochemical and environmental fate (E-Fate) studies. Helping your lab to achieve productivity goals and GLP confidence, whilst avoiding transcription errors. Find out more.
Debra 6.3.1 Release
12/02/2017
LabLogic are pleased to announce the latest version 6.3.1 has just been released.
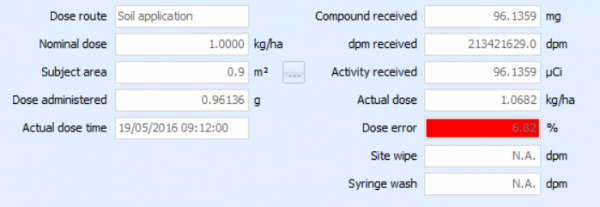
Debra's 'topical' study type
25/05/2016
Whether you are performing dermal skin studies or applying compounds to soil or crops, Debra 6.3 now has a 'topical' study type with convertible dose units ranging from ng/cm3 to g/m3 up to kg/ha.
Debra 6.3 Release
28/01/2016
The 6.3 version of Debra will be released in Feburary with a number of new features including: Audited and versioned rich text comments, new topical dose study types, TRR calculations, extraction tree comments and enhanced links.

LabLogic’s latest release of Debra
12/03/2015
LabLogic is pleased to announce the latest release of the industry standard LIMS for radiolabelled metabolism studies, Debra.
Latest Debra release incorporates a new module for environmental fate studies
21/02/2014
For users of Debra who are looking more at environmental fate studies, the latest Debra 6.1 release incorporates the previously standalone system Trace as part of its core functionality.
Certara partners With LabLogic Systems to facilitate faster drug discovery analyses
10/12/2013
Customers can now import ADME and protein-binding data directly from Debra to Phoenix
Certara™, a leading provider of software and scientific consulting services to improve productivity and decision-making from drug discovery through drug development, has announced that it has partnered with LabLogic Systems to integrate Certara’s Phoenix® WinNonlin® pharmacokinetic (PK)/pharmacodynamic (PD) modeling and non-compartmental analysis product with LabLogic’s Debra™ Laboratory Information Management System (LIMS). Debra is used to manage data from absorption, distribution, metabolism, and excretion (ADME) and protein-binding studies. The resulting solution, allows customers who are using Debra 6 as their LIMS for drug metabolism studies, to push their data directly into Phoenix WinNonlin.
QPS implements DEBRA LIMS for Radiolabelled Metabolism Studies
20/08/2013
QPS a leading preclinical and clinical CRO, has now fully validated LabLogic Debra version 6.0.6 for use in radiolabelled metabolism studies. An earlier version was implemented in 2010 to support discovery stage radiolabel ADME studies. The implementation of Debra LIMS coupled with the recent addition of a high resolution mass spectrometer (HRMS) ensures rapid integration of radioactivity data from mass balance excretion and/or quantitative whole-body autoradiography (QWBA) studies with metabolite identification and radio-quantitation to shorten timelines in support of preclinical and human mass balance studies.
Promising first year for Debra 6
17/01/2013
One year on since the launch of Debra 6, LabLogic are pleased to report that 3 sites have upgraded to the latest release with another brand new customer and several additional sites currently evaluating the system with a view to upgrading early this year.
Charles River upgrade to the latest LabLogic Debra software
28/05/2012
LabLogic, a leading provider of drug metabolism LIMS and radiochromatography solutions has announced today that Charles River has upgraded to Debra version 5.7.10 for its radiolabelled mammalian metabolism and environmental fate studies. Charles River has been using LabLogic’s software for over 13 years for the management of all chemicals and pharmaceutical ADME studies. This upgrade follows on from the recent upgrade of the chromatographic system Laura to version 4.1.4.
Quotient upgrade to the latest LabLogic software
27/03/2012
LabLogic is pleased to announce that Quotient Bioresearch has upgraded to the latest Debra version 5.7.10 and Laura version 4.1.3 platforms for its radiolabelled metabolism studies and radiochromatography work.
Software demonstrations made easy
16/11/2011
LabLogic Systems has made it easier than ever to see demonstrations of its software for drug metabolism, PET and radio-chemistry.
New version of ADME LIMS to be unveiled at AAPS meeting
21/10/2011
LabLogic Systems will launch Version 6 of its Debra LIMS for drug metabolism studies at the American Association of Pharmaceutical Scientists 2011 Annual Meeting and Exposition in Washington DC from 23 to 27 October.
Debra LIMS featured in CRO data management debate
03/03/2011
A user of LabLogic’'s Debra drug metabolism LIMS has contributed to an article in the February/March 2011 issue of ‘Scientific Computing World’ magazine on the subject of flexible data management solutions for contract laboratories.
ADME LIMS on show at Society of Toxicology 50th anniversary meeting
17/02/2011
Experts from LabLogic Systems will be at the Society of Toxicology’s 50th anniversary meeting to explain the advantages of Debra, the LIMS that has been setting the standards for drug metabolism and ADME for almost two decades.
ADME LIMS will accelerate data reporting at QPS
01/11/2010
The newest customer for Debra, LabLogic’s LIMS for absorption, distribution, metabolism and excretion studies (ADME), is GLP/GCP-compliant contract research organisation QPS, which recently installed the latest 5.7.8 version at its Newark, DE facility.
Transatlantic CRO adopts common radio-labelled ADME platform
05/08/2010
A US/UK contract research laboratory is standardising on Debra 5.7.8, the latest release of LabLogic’'s drug metabolism LIMS, as a common platform for its radio-labelled ADME studies.
Latest release makes drug metabolism LIMS even easier to use
30/03/2010
Greater flexibility in reporting data and additional features requested by users predominate in the new 5.7.7 release of Debra, LabLogic Systems’ market-leading LIMS for ADME studies.
New ADME LIMS release makes protein binding easier
27/10/2009
Debra 5.7.6, the latest release of LabLogic’s LIMS for drug metabolism studies, implements more than 70 modifications.
LabLogic maximises searchability of pharma's LIMS
04/09/2009
LabLogic Systems has helped a major biopharmaceutical company to create a Pipeline Pilot reporting tool that can query for study data held in its Debra drug metabolism LIMS and deliver a customised report in a form that is meaningful to users.
Drug metabolism LIMS in demand for clinical studies
23/06/2009
LabLogic Systems reports a rapid rise in the number of clinical studies being performed using its drug metabolism LIMS Debra.
Is your LIMS working hard enough?
17/03/2009
Pharmaceutical industry researchers grappling with the implications of recent staff cutbacks need to make sure that their software systems are contributing fully to the task of getting more results from fewer resources.
Latest pharma software on show at SOT meeting
23/02/2009
LabLogic's laboratory information management system and chromatography software for the pharmaceutical metabolism industry will be to the fore at the 48th Annual Meeting of the Society of Toxicology (15th -19th March, Baltimore, USA).
XenoBiotic Laboratories chooses Debra LIMS
23/01/2009
Contract research organisation XenoBiotic Laboratories, Inc. (XBL) has purchased LabLogic Systems' Debra LIMS solution to manage ADME data at its New Jersey research facility.
User feedback tunes up new release of ADME LIMS
08/01/2009
LabLogic Systems already has six sites validating Debra 5.7, the latest release of its LIMS for non-clinical ADME studies.
CRO chooses flexible LIMS user licence
29/09/2008
Kansas-based contract laboratory Xenometrics LLC is taking advantage of the new flexible licensing options available for Debra, LabLogic Systems' LIMS for non-clinical ADME studies.
ADME LIMS suitable for unlabelled compound data
05/09/2008
Millennium Pharmaceuticals of Cambridge, Massachusetts is the latest company to install LabLogic’s Debra LIMS.
Latest Debra has Protein Binding module
28/07/2008
A new Protein Binding module is available with Debra 5.7, the latest release of LabLogic’s ADME LIMS.
ADME LIMS keeps an eye on sampling time deviations
18/07/2008
The latest release of Debra, LabLogic Systems' LIMS for non-clinical ADME studies, makes it even easier to collect and monitor time data for blood PK samples.
Keeping tabs on radioisotopes - and more
08/07/2008
Recent new users of Stacy, the sample management and radioisotope inventory system from LabLogic Systems, are still finding new applications for the software.
User Group calls for radiochromatography / LIMS papers
25/03/2008
LabLogic Systems is calling for papers for its 2008 LIMS and Radiochromatography User Group, to be held at the San Diego Hilton on the 9th and 10th October.
LabLogic/INUS 2008 LIMS and Radiochromatography User Group
12/02/2008
We are pleased to confirm the dates and location for our 2008 LIMS and Radiochromatography User Group. The event will be held at the San Diego Hilton, San Diego, USA, on the 9th and 10th October 2008.
Debra times six for Sanofi-Aventis ADME research
08/11/2007
Sanofi-Aventis has adopted LabLogic's Debra LIMS for non-clinical ADME studies across all six of its R&D locations in Europe and North America."
UCB chooses LabLogic software for Brussels R&D centre
09/10/2007
UCB is the latest pharmaceutical company to invest in software for drug metabolism studies from LabLogic Systems.
Debra User Group 2000
09/08/2000
25th - 28th April 2000, joint Debra and Watson User Group, PA, USA. Catch up on this years topics and presentations.